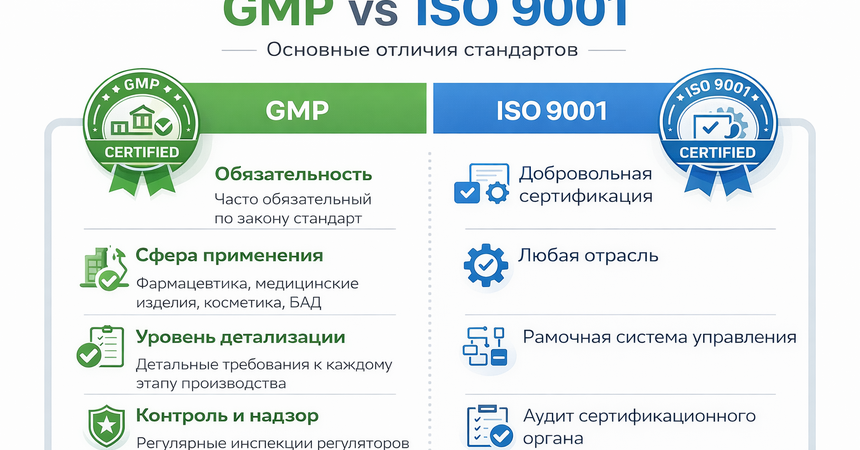
Manufacturing companies often need to choose between different quality standards. The most common ones are GMP and ISO 9001, along with other management systems. Although they share similar goals, these standards differ significantly.
What Is GMP
GMP (Good Manufacturing Practice) is a mandatory set of requirements focused on ensuring product safety and consistent quality. GMP strictly regulates manufacturing processes, facilities, equipment, personnel, and documentation.
GMP applies to regulated industries where errors can directly affect consumer health and safety.
What Is ISO 9001
ISO 9001 is an international quality management system standard applicable to organizations in any industry. It focuses on process management, customer satisfaction, and continuous improvement.
ISO 9001 does not define specific manufacturing conditions but provides a general management framework.
Key Differences Between GMP and ISO 9001
Main differences include:
• Mandatory nature- GMP - often legally required
- ISO 9001 - voluntary certification
- GMP - pharmaceuticals, medical devices, cosmetics, supplements
- ISO 9001 - all industries
- GMP - detailed production and compliance requirements
- ISO 9001 - high-level management framework
- GMP - inspections by regulatory authorities
- ISO 9001 - audits by certification bodies
GMP is also compared with:
- ISO 14001 - environmental management
- SO 45001 - occupational health and safety
- HACCP - food safety management
- GDP - good distribution practice
Can GMP and ISO Be Combined
In practice, GMP and ISO 9001 are often implemented together:
- ISO 9001 builds a structured management system
- GMP ensures compliance with regulatory requirements
How to Choose the Right Standard
The choice depends on:
- industry sector
- regulatory requirements
- target markets
- business strategy
Conclusion
GMP and ISO 9001 serve different purposes. GMP ensures product safety and legal compliance, while ISO 9001 enhances operational efficiency. In most cases, implementing both standards provides the best results.
If you need support with GMP, ISO 9001, or other management systems, contact WorldWideBridge - we will deliver a professional turnkey solution tailored to your business.