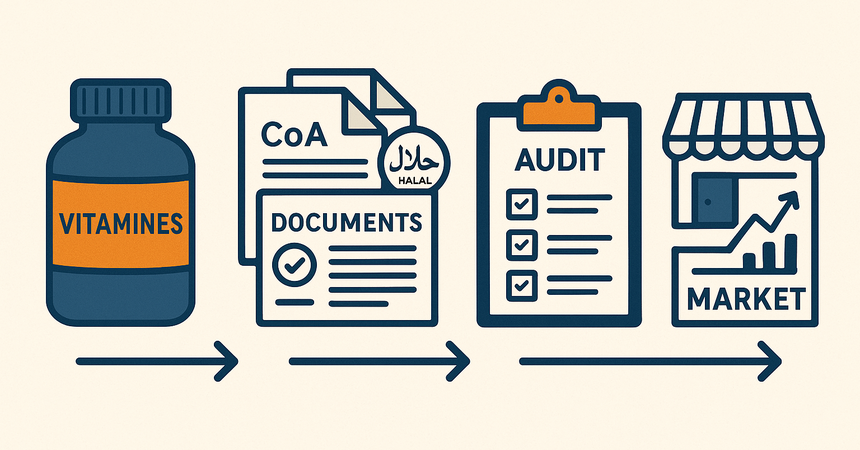
In recent years, Morocco has seen steady growth in demand for vitamins and nutraceuticals — from consumers, pharmacy chains, retail, and online channels alike. Despite similarities with other MENA countries, the Moroccan market has specific regulatory nuances that every manufacturer should understand.
What Is Considered a Nutraceutical in Morocco?
Morocco has no legal definition for "nutraceutical." The term generally covers:- Vitamins and multivitamin-mineral complexes
- Amino acids
- Omega-3, collagen, coenzyme Q10
- Products supporting immunity, joints, metabolism, energy
- Natural extracts with proven effects (curcumin, resveratrol, ginseng, etc.)
Depending on composition and claims, a product may be classified as:
- Dietary supplement (complément alimentaire)
- Pharmaceutical drug
- Functional food product
This classification defines the registration pathway.
Key Regulatory Authorities
- ONSSA (Office National de Sécurité Sanitaire des Produits Alimentaires)
- DMP (Direction du Médicament et de la Pharmacie)
- IMANOR
What’s Required for Registration?
For standard vitamins and nutraceuticals classified as compléments alimentaires, the following documents are typically required:1. Technical Dossier:
- Full ingredient list with dosages
- Manufacturing process description
- Safety and efficacy rationale (for active components)
2. Manufacturer Certificates:
- GMP / ISO 22000 / HACCP
3. Free Sale Certificate
4. Certificate of Analysis (CoA)
- For each active substance
- 5. Labeling and packaging mockup in French
- o Including dosage, format, storage instructions
6. Halal Certificate
- o Required if animal-derived components are used
Limitations and Checks
- Daily intake levels must comply with Moroccan or international RDI (Recommended Daily Intake) standards
- Some forms (e.g., retinoids or megadoses of vitamin D) may be considered drugs
- Restricted or prohibited ingredients include:
- Melatonin
- Synephrine
- DHEA
- Unapproved stimulants
Registration Timeline
- Pre-registration dossier audit: 1–2 weeks
- Submission and review (ONSSA or DMP): 2–4 months
- Additional queries and corrections: 2–4 weeks
- Total: approx. 3–5 months, depending on product classification
Risks and Challenges
- Ambiguous classification for products with antioxidant or hormone-like effects
- Customs may request extra documentation even with approvals
- Registration is not possible without a local representative or importer
How to Simplify Market Entry
To avoid delays and refusals:- Conduct a preliminary assessment of ingredients and marketing claims
- Adapt labels and promotional texts to local norms
- Appoint a local distributor or authorized representative
- Ensure Halal certification, especially for products in capsules, with omega, or collagen
WorldWideBridge — Your Expert in Moroccan Nutraceutical Registration
We help you:- Analyze formulation and dosages
- Prepare the dossier and adapt documentation
- Register through ONSSA or DMP
- Obtain Halal certification
- Find a local representative and distributor
- Support your product from start to market launch